LAUROMACROGOL 100
Outline
Polyoxyethylene Lauryl Alcohol Ether, Corresponding to the Japanese Pharmacopoeias, For Exclusive Use in Manufacturing of Pharmaceutical Products
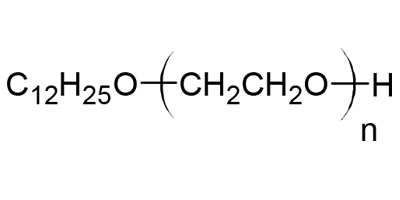
Features
· Polyoxyethylene lauryl alcohol ether, which conforms to LAUROMACROGOL listed in the Japanese Pharmacopoeia.
· It has been approved in Japan as a medical drug for exclusive use in manufacturing.
· It is manufactured by adding ethylene oxide (EO) to plant-derived lauryl alcohol using our original Narrow Range Ethoxylation. This made it possible for LAUROMACROGOL 100 to have a low lauryl alcohol content and narrow EO-adduct mole distribution. Therefore, this product has high surface activity.
· Exhibits an excellent surface tension lowering property, penetrating property and detergency because this product has a low lauryl alcohol content and narrow EO-adduct mole distribution.
· Can be blended with anionic and cationic surfactants at any desired ratio.
Functions
- ・Cleaning
- ・Wetting/Penetrating
Applications
- ・Pharmaceuticals/Quasi-Drugs/Medical Supplies
Composition
Nonionic Surfactants Ether-based
Properties, and other information
| Appearance | Pale yellow petrolatum jelly | pH(Sample Concentration) | 6.5(1 wt % aqueous solution) | HLB | 14 |
| Ionic | Nonionic |
Precaution Against Mishandling
· LAUROMACROGOL 100 is a nonionic surfactant. If this product is used together with sodium hypochlorite as a bleaching agent, the sodium hypochlorite will decompose. In addition, this product will be oxidized and its performance will degrade. Therefore, do not use sodium hypochlorite as the bleaching agent
Before handling this product, refer to the current Safety Data Sheet for recommended protective equipment, and detailed precautionary and hazards information.If SDS is not listed on the homepage, please contact our sales representative.
