Provision of safe and secure products
By communicating with customers, we understand their needs in terms of performance, quality, and the environment, introduce products and develop new products that meet those needs, and provide appropriate technical and safety information concerning our products.
For newly marketed products, we have employed a system to start sales only after we understand the intended use of the customers and confirm the compliance of the products with the customers’ requirements, including green procurement and use of non-conflict minerals. We also set a rule to eliminate chemical substances that do not comply with the customers’ needs early in the product design stage.
Product liability (PL)
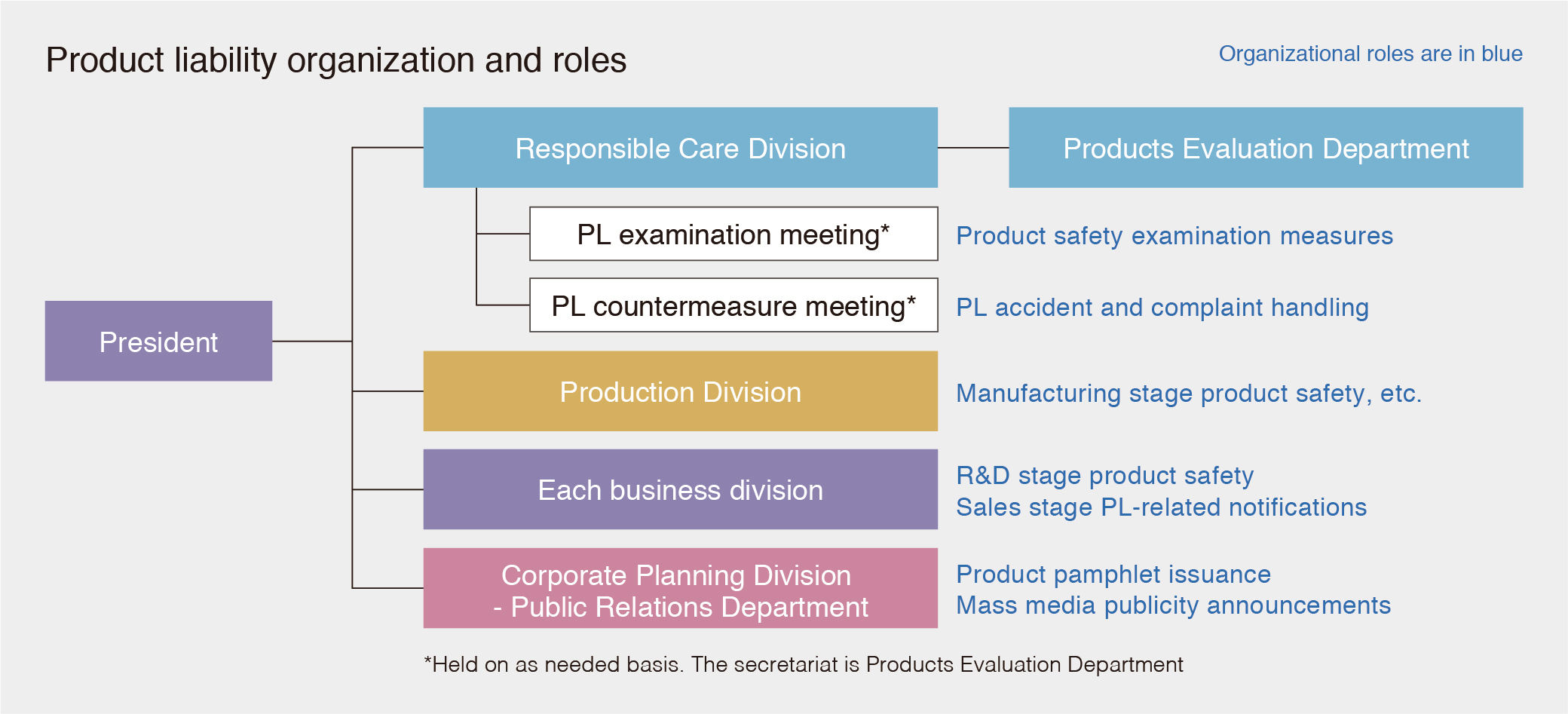
In accordance with our Basic Product Liability (PL) Code, we set up a dedicated department (Products Evaluation Department) concerning PL to prevent PL-related accidents. The Products Evaluation Department oversees examination of products for compliance with relevant laws and regulations in Japan and overseas, examination of labels and safety data sheets (SDS), our management of chemical substances, and our response to customers.
In the event of PL-related deliberations, accidents or complaints, we will convene an investigative meeting and a countermeasure meeting to deal with them accordingly.
Provision of product safety information

When we introduce our products via a pamphlet or other media, we also submit the SDS at the same time so that the customer can check the technical features and the safe handling methods. Following the revision of laws related to chemical substances, including the Chemical Substance Control Law, Industrial Safety and Health Act, PRTR Law, and Poisonous and Deleterious Substances Control Act, we revised the SDS and product label accordingly. To each driver of trucks and tanker trucks that transport our products, we issue a yellow card with first-aid methods and emergency contact information so that they can appropriately respond in case of an accident during transportation.
Export control
The export of chemical products may be restricted due to the Export Trade Control Order in Japan, laws/regulations of destination countries, or international treaties. In order to observe those rules, we have incorporated an export stop system in our order system. When making entry of an order, the system rejects products not registered in our export control database. Products Evaluation Department closely examines and inputs information into the database serving as the foundation of this system, such as product chemical compositions and their status in other countries and any restrictions imposed by any laws or regulations. The Export Administration Committee discusses and examines our group’s compliance to laws and regulations in concerned countries and then implements changes as necessary.
Positive quality control
We produce and market approximately 3,000 types of performance/chemical products. We have automated the processes as much as possible through adaptive plant design for variable multi-variety production.
Our domestic and overseas factories have acquired ISO 9001 certification and built quality control systems in synergy with the quality control system of our company.
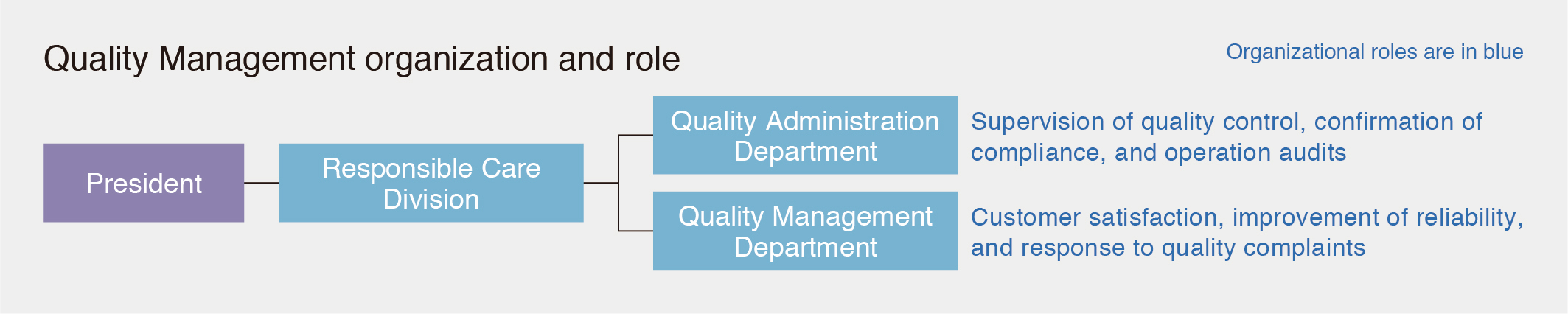
Quality assurance system
Product quality judgment and stability, and customer’s complaints and inquiries had been managed by the Quality Assurance Section of the corresponding factory. In order to have independence from the Production Division, we transferred these functions to the Responsible Care Division in April 2022.
In case quality issues occur, we will immediately investigate the cause, draft recurrence prevention measures, promptly respond to customers, and share the information concerning the issue among group factories to horizontally develop countermeasures.
Medicine and medical device quality assurance
As a pharmaceutical-related quality control management system, the Biotechnology & Medical Division acquired ISO 13485 certification for medical devices in May 2019 and for invitro diagnostic medicine in May 2021. In response to the partially revised Pharmaceutical Affairs Law on August 1, 2021, in the Biotechnology & Medical Division, in addition to the Pharmaceutical Affairs Department at our Kyoto Factory, we established a Pharmaceutical Affairs Department at our Nagoya Factory in January 2022.
With customers
We are striving to disseminate our new product and technical information through press releases, press conferences, and other means, as well as direct communications with customers at exhibitions and wherever possible. On the product search page of our website, it is possible to search not only by function, application, and composition, but also by social needs.

In April 2022, we launched our website to introduce resin/functional chemicals and explain our product/technical information. The website contains detailed information to deepen understanding of our performance chemicals, such as each product’s features and use applications, explanations of functions, and other basic knowledge.
Products&Technologies site:https://sanyo-chemical-solutions.com/